FDA Announces Recall of Specific Miniverse Toys Due to Health-damaging Acrylates
30 November 2024, Quezon City. The toxics watchdog group EcoWaste Coalition gave the Food and Drug Administration (FDA) a thumbs up for issuing a toy safety advisory that will protect children from being harmed by acrylates in liquid form, which pose a safety risk to consumers, especially kids.
The FDA through Advisory No. 2024-1621 issued on November 28 and posted on the agency’s website on November 29 informed the public about the voluntary recall of 15 specific types of Miniverse mini toys for containing hydroxyethyl methacrylate (HEMA) and isobornyl acrylate (IBOA) at levels prohibited under the US Federal Hazardous Substances Act.
“We commend the FDA for coordinating with the market authorization holder (MAH) towards the voluntary recall of affected stocks of Miniverse mini toys,” said Aileen Lucero, National Coordinator, EcoWaste Coalition. “We likewise laud the MAH for taking steps to ensure that the recalled products are not sold in the market, including in online shopping platforms.”

.
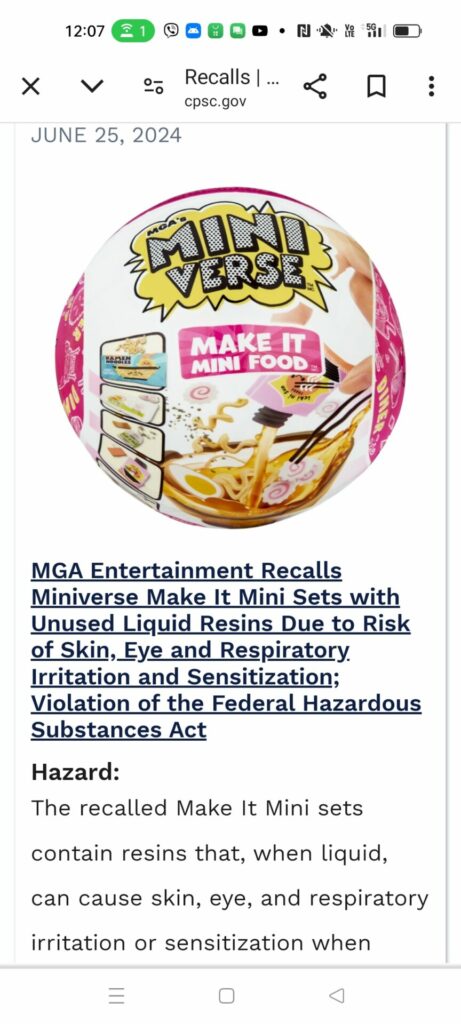
Screenshots of Miniverse Make It Mini Food being recalled in USA and Canada.
The EcoWaste Coalition last June 29 wrote to the FDA about the massive recall of some 22 million units of Miniverse mini toys in Canada and the USA. In the said letter, the group requested the FDA to reach out to, Ban Kee Trading, Inc., the MAH, to determine if a similar recall is warranted for certain items being offered for sale in the Philippines.
According to the US Consumer Product Safety Commission and the MGA Entertainment based in California, USA, which imported the products from China, the recalled toy sets have unused liquid resins “when in liquid form or part of an uncured creation can cause skin, eye, and respiratory irritation or sensitization when inhaled, touched, or ingested by children or adults.” Prior to the recall, MGA had received reports of skin burns and irritation and respiratory irritation due to exposure to the liquid resins.
As stated in the said FDA advisory, the recall is limited to specific item/model/stock-keeping unit (SKU) numbers, which are as follows:
- Miniverse – Mini Home S1B (Item/Model/SKU No. 591856)
- Miniverse – Mini Foods Diner S2B (Item/Model/SKU No. 591825)
- Miniverse – Mini Foods Cafe S2B (Item/Model/SKU No. 591818)
- Miniverse – Make It Mini: Holiday (Item/Model/SKU No. 593782)
- Miniverse – Make It Mini: Home Series 1A (Item/Model/SKU No. 591856)
- Miniverse – Make It Mini: Multi-pack (Item/Model/SKU No. 591849)
- Miniverse – Make It Mini: Kitchen (Item/Model/SKU No. 591832)
- Miniverse – Make It Mini Foods: Diner in S2A (Item/Model/SKU No. 591825)
- Miniverse – Make It Mini Foods: Cafe in S2A (Item/Model/SKU No. 591818)
- Miniverse – Food/Dinner (Item/Model/SKU No. 589938)
- Miniverse – Food Series Cafe (Item/Model/SKU No. 587200)
- Miniverse – Mini Appliances (Item/Model/SKU No. 505600)
- Miniverse – Make It Mini Foods: Diner in PDQ Series 3A (Item/Model/SKU No. 505419)
- Miniverse – Make It Mini Foods: Cafe in PDQ Series 3A (Item/Model/SKU No. 505396)
- Miniverse – Make It Mini Lifestyle Home S1A (Item/Model/SKU No. 505372)
Through the advisory, the FDA warned all concerned establishments not to distribute the above-listed stocks of Miniverse. It also advised consumers not to purchase the said toys.
The FDA requested its regional field offices and regulatory enforcement units, law enforcement agencies and local government units to ensure that the affected stocks of Miniverse mini toys will no longer be made available in the market within their areas of jurisdiction.
“Consumers who may have purchased the above-mentioned products may direct their questions and/or report any adverse reactions from the use of the product to Ban Kee Trading, Inc. through chrisvan.bankee@
References:
https://www.fda.gov.ph/wp-content/uploads/2024/11/FDA-Advisory-No.2024-1621-20240920094336.pdf
https://www.cpsc.gov/Recalls/2024/MGA-Entertainment-Recalls-Miniverse-Make-It-Mini-Sets-with-Unused-Liquid-Resins-Due-to-Risk-of-Skin-Eye-and-Respiratory-Irritation-and-Sensitization-Violation-of-the-Federal-Hazardous-Substances-Act






